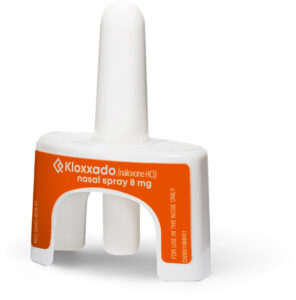
On April 30, 2021, the US Food and Drug Administration approved of KLOXXADO® (naloxone HCl) Nasal Spray 8 mg, a new opioid overdose reversal medication.
Naloxone, the key medicine in KLOXXADO®, has a long history of safe use as the standard of care for reversing opioid overdoses. It can be administered by individuals with or without medical training. According to health organizations, widely prescribing and distributing naloxone may play a vital role in the fight against opioid overdose.
“Communities are looking for tools to respond to the epidemic of drug overdoses, and the FDA action today adds a powerful one,” said Dr. Patrice Harris, chair of the American Medical Association’s opioid task force.
The FDA approval of KLOXXADO® is a vital step in providing patients, friends and family members – as well as the public health community – with a new option for treating opioid overdose.
KLOXXADO® is sold in packages containing two nasal spray devices and is now available in pharmacies and community programs.
Please see the full Prescribing Information and Medication Guide for KLOXXADO® for complete product details.
NOTE: This article was not written by a medical professional and is not intended to substitute for the guidance of a physician. These are not Emergent’s recommendations, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.