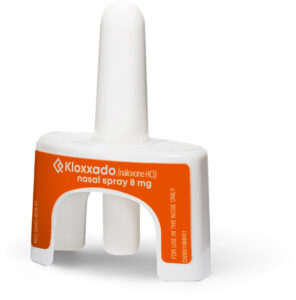
Hikma Pharmaceuticals announced, on April 30, 2021, the US Food and Drug Administration (FDA) approval of Kloxxado® (naloxone HCl) Nasal Spray 8 mg, a new opioid overdose reversal medication. This announcement came as drug overdose deaths continued to increase during the COVID-19 pandemic.1
Naloxone, the key medicine in Kloxxado®, has a long history of safe use as the standard of care for reversing opioid overdoses.2 It can be administered by individuals with or without medical training. According to health organizations, widely prescribing and distributing naloxone may play a vital role in the fight against opioid overdose.3
However, with the increasing prevalence of potent, illegally-manufactured synthetic opioids, more naloxone may be required to keep a patient alive until medical help arrives or can be transferred to a medical facility.3 Kloxxado® contains twice as much naloxone per spray as Narcan® (naloxone HCl) Nasal Spray 4 mg.4,5
“Communities are looking for tools to respond to the epidemic of drug overdoses, and the FDA action today adds a powerful one,” said Dr. Patrice Harris, chair of the American Medical Association’s opioid task force. “The FDA is making sure the overdose-reversing drug is potent enough to counteract the increasingly lethal and illicitly manufactured fentanyl.”
The FDA approval of Kloxxado® is a vital step in providing patients, friends and family members – as well as the public health community – with a new option for treating opioid overdose.
Kloxxado® is sold in packages containing two nasal spray devices and is now available in pharmacies and community programs.
For more details, see the latest news coverage:
FDA statement on the approval of Kloxxado®
ABC News: US approves high-dose opioid reversal nasal spray from Hikma
AMA Statement on the approval of Kloxxado®
Kloxxado® is a trademark of Hikma Pharmaceuticals USA Inc.
Narcan® is a registered trademark of Emergent Operations Ireland Limited.
Important Safety Information About Kloxxado® (naloxone HCl) Nasal Spray
Contraindications
Hypersensitivity to naloxone hydrochloride or to any of the other ingredients
Warnings and Precautions
- Use Kloxxado® right away if you suspect an opioid overdose emergency, even if you are not sure, because an opioid overdose emergency can cause severe injury or death. Signs and symptoms of an opioid overdose emergency may include:
- Unusual sleepiness; you are not able to awaken the person with a loud voice or by rubbing firmly on the middle of their chest (sternum).
- Breathing problems, including slow or shallow breathing in someone difficult to awaken or who looks like they are not breathing.
- The black circle in the center of the colored part of the eye (pupil) is very small (sometimes called “pinpoint pupils”) in someone difficult to awaken.
- Family members, caregivers or other people who may have to use Kloxxado® in an opioid overdose emergency should know where Kloxxado® is stored and how to give Kloxxado® before an opioid overdose emergency happens.
- Get emergency medical help right away after using the first dose of Kloxxado®. Rescue breathing or CPR (cardiopulmonary resuscitation) may be needed while waiting for emergency medical help.
- The signs and symptoms of an opioid overdose emergency can return after Kloxxado® is given. If this happens, give another dose after 2 to 3 minutes, using a new Kloxxado® device, alternating nostrils, and watch the person closely until emergency medical help arrives.
- Do not use Kloxxado® if you are allergic to naloxone hydrochloride or any of the ingredients in Kloxxado®.
- Kloxxado® can cause sudden and severe opioid withdrawal, the symptoms of which may include body aches, diarrhea, increased heart rate, fever, runny nose, sneezing, goosebumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, stomach cramps, weakness and increased blood pressure.
- In infants, opioid withdrawal may be life-threatening if not recognized and properly treated. Infants going through opioid withdrawal may have seizures, cry more than normal, and have increased reflexes.
- Tell your doctor about all of your medical conditions before using Kloxxado®, including if you have heart problems, are pregnant or plan to become pregnant.
- Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, drugs, vitamins and herbal supplements.
Side Effects
The following serious side effect is discussed in the full Prescribing Information for Kloxxado®:
- Sudden and Severe Opioid Withdrawal
Symptoms of sudden and severe opioid withdrawal resulting from the use of Kloxxado® in someone regularly using opioids include: body aches, diarrhea, increased heart rate, fever, runny nose, sneezing, goosebumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, stomach cramps, weakness and increased blood pressure.
Infants may have seizures, cry more than normal and have increased reflexes.
Some people may become aggressive after abrupt reversal of opioid overdose.
In two clinical studies, a total of 47 healthy adult volunteers were exposed to a single dose of Kloxxado®, one spray in one nostril. Side effects were reported in two subjects for each of the following: abdominal pain, asthenia, dizziness, headache, nasal discomfort, and presyncope.
These are not all of the possible side effects of Kloxxado®. Contact your doctor for medical advice about side effects.
Pregnancy, Infancy and Breastfeeding, Children
Tell your doctor if you are pregnant or plan to become pregnant. If you are pregnant and opioid dependent, use of Kloxxado® may cause withdrawal symptoms in you and your unborn baby. A healthcare provider should monitor you and your unborn baby right away after you use Kloxxado®.
There is no information regarding the presence of naloxone in human milk, the effects of naloxone on the breastfed infant or on milk production.
If the primary concern is an infant at risk of an overdose, consider whether other naloxone-containing products may be more appropriate.
Kloxxado® nasal spray is safe and effective in children for known or suspected opioid overdose.
Dosage and Administration
Do not attempt to prime or test-fire the device. Each Kloxxado® Nasal Spray contains only 1 dose of medicine and cannot be reused. Read the “instructions for use” at the end of the Prescribing Information and Medication Guide for detailed information about the right way to use Kloxxado® Nasal Spray.
Storage and Handling
Store Kloxxado® at room temperature between 59 F to 77F (15C to 25C). Kloxxado® may be stored for short periods between 39°F to 104°F (4°C to 40°C). Do not store above 40ºC (104ºF). Do not freeze Kloxxado®. Keep Kloxxado® in its box until ready to use. Protect from light. Replace Kloxxado® before the expiration date on the box. Keep Kloxxado® and all medicines out of the reach of children.
Please see the full Prescribing Information and Medication Guide for Kloxxado® for complete product details.
NOTE: This article was not written by a medical professional and is not intended to substitute for the guidance of a physician. These are not Hikma’s recommendations, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.
HK-1299
References
- Kamp, Jon, Campo-Flores, Arian. The Opioid Crisis, Already Serious, Has Intensified During Coronavirus Pandemic. The Wall Street Journal, Sept. 2020. https://www.wsj.com/articles/the-opioid-crisis-already-serious-has-intensified-during-coronavirus-pandemic-11599557401?mod=article_inline/. Accessed July 20, 2021
- Jordan, M.R. and Morrisonponce, D. 2021, “Naloxone,” StatPearls Publishing, https://pubmed.ncbi.nlm.nih.gov/28722939/. Accessed May 3, 2021.
- CDC Health Network. Increase in Fatal Drug Overdoses Across the United States Driven by Synthetic Opioids Before and During the COVID-19 Pandemic. https://emergency.cdc.gov/han/2020/han00438.asp.
- KLOXXADO® (naloxone HCl) Nasal Spray [prescribing information]. Columbus, OH: Hikma Specialty USA Inc., 2021
- NARCAN® (naloxone HCl) Nasal Spray [prescribing information]. Plymouth Meeting, PA: Emergent BioSolutions Inc., 2020